Mastering Electron Energy and Light: A Comprehensive Guide with POGIL Activities
The fascinating dance between electrons and light governs everything from the vibrant colors of a rainbow to the inner workings of solar panels. Understanding the relationship between electron energy levels and the emission of light is fundamental to grasping concepts in chemistry, physics, and even materials science. This article provides a comprehensive overview of this crucial topic, incorporating the benefits of Process Oriented Guided Inquiry Learning (POGIL) activities to enhance your comprehension.
What is Electron Energy and Why Does it Matter?
Electrons, the tiny negatively charged particles that orbit the nucleus of an atom, don’t just zip around randomly. They exist in specific energy levels, often visualized as distinct “shells” or “orbits” around the nucleus. These energy levels are quantized, meaning electrons can only occupy specific, discrete energy states, much like steps on a staircase.
- Key Concepts:
- Energy Levels: Electrons exist in specific energy levels, determined by their distance from the nucleus. Lower levels (closer to the nucleus) have lower energy.
- Quantization: Electrons can only have specific, defined amounts of energy. They can’t exist with energy values in between these levels.
- Excitation: When an atom absorbs energy (e.g., from heat or light), an electron can jump to a higher energy level (excited state).
- Relaxation/Emission: An excited electron is unstable and will quickly “fall” back down to a lower energy level, releasing the excess energy as a photon of light.
Understanding electron energy is critical because it explains:
- Atomic Spectra: The unique patterns of light emitted by different elements.
- Chemical Bonding: How atoms interact to form molecules.
- Material Properties: The color, conductivity, and other characteristics of materials.
- Photovoltaics: The operation of solar cells and other light-based technologies.
The Role of Light: Photons and Electromagnetic Radiation
Light is not just a continuous wave; it also behaves as a stream of particles called photons. Each photon carries a specific amount of energy, directly related to its wavelength and frequency.
- Key Concepts:
- Photon Energy: The energy of a photon (E) is directly proportional to its frequency (ν) and inversely proportional to its wavelength (λ). The relationship is defined by the equation:
E = hν = hc/λwhere ‘h’ is Planck’s constant and ‘c’ is the speed of light. - Electromagnetic Spectrum: Light encompasses a broad spectrum of wavelengths, from radio waves (low energy, long wavelength) to gamma rays (high energy, short wavelength).
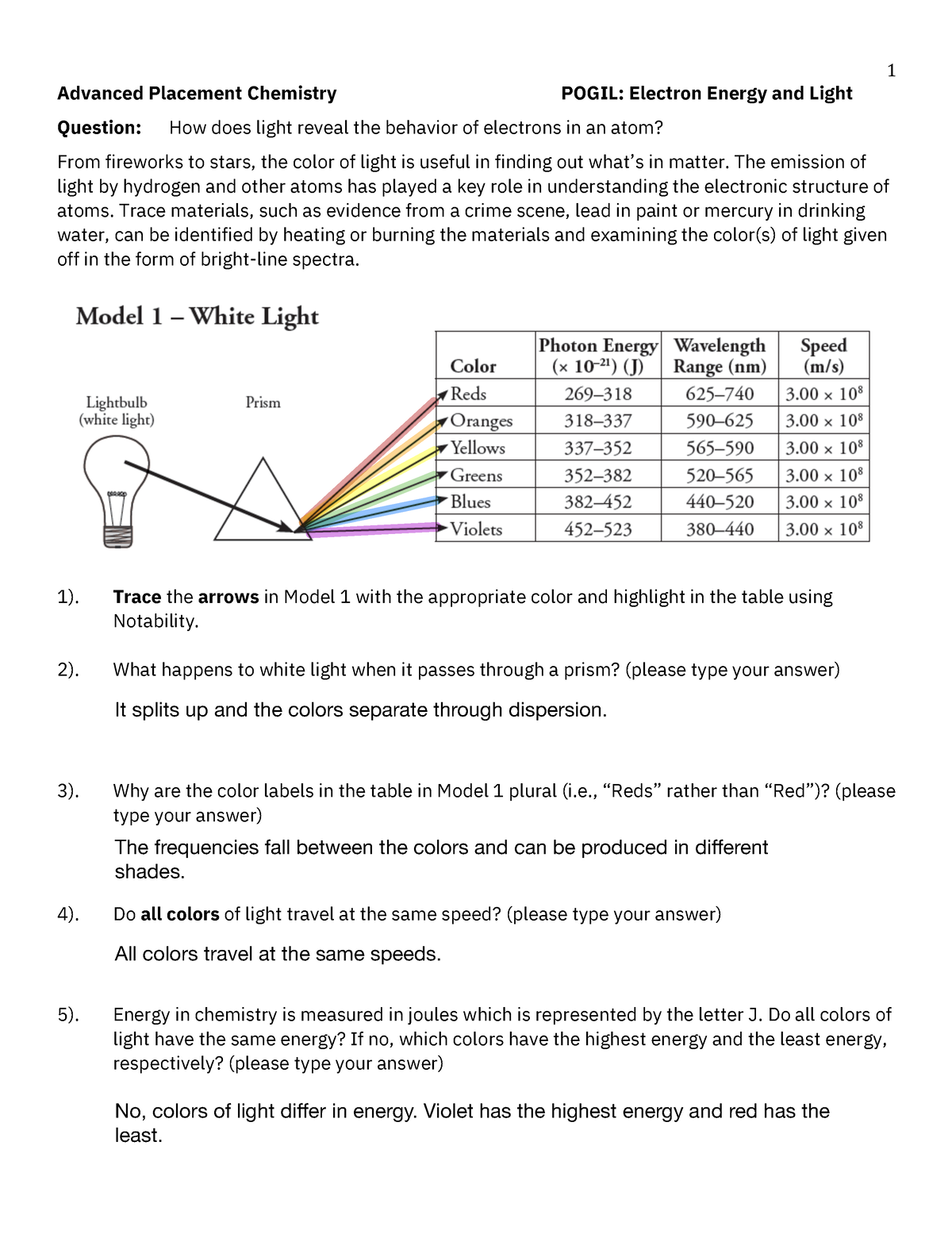
- Color and Energy: Visible light is a small portion of the electromagnetic spectrum. Each color of light corresponds to a specific energy and wavelength. Red light has lower energy and longer wavelengths compared to blue light.
- Photon Energy: The energy of a photon (E) is directly proportional to its frequency (ν) and inversely proportional to its wavelength (λ). The relationship is defined by the equation:
The energy of a photon must match the energy difference between electron energy levels for an electron to absorb or emit it. This precise matching is why each element has a unique atomic spectrum.
POGIL Activities: Deepening Understanding Through Inquiry
Process Oriented Guided Inquiry Learning (POGIL) is an active learning approach that encourages students to construct their own understanding of concepts through guided exploration and collaboration. POGIL activities are particularly effective for grasping complex topics like electron energy and light.
- Benefits of POGIL:
- Active Learning: Students are actively involved in the learning process, not just passively receiving information.
- Collaboration: Students work together in small groups to analyze data, answer questions, and solve problems.
- Critical Thinking: POGIL activities promote critical thinking and problem-solving skills.
- Conceptual Understanding: Students build a deeper understanding of the underlying concepts, rather than just memorizing facts.
Examples of POGIL Activities for Electron Energy and Light:
- Analyzing Atomic Spectra: Students analyze spectral data from different elements to identify the wavelengths of light emitted and relate them to electron energy transitions.
- Modeling Electron Energy Levels: Students use diagrams or models to visualize electron energy levels and the process of excitation and emission.
- Calculating Photon Energy: Students practice applying the equation
E = hν = hc/λto calculate the energy of photons and relate it to the color of light. - Investigating the Photoelectric Effect: Students explore the relationship between light and the emission of electrons from a metal surface.
Applying the Concepts: Examples and Applications
The understanding of electron energy and light is crucial in various fields:
- Spectroscopy: Analyzing the absorption and emission of light by substances to identify their composition and structure.
- Lasers: Devices that produce highly focused beams of light based on the controlled emission of photons from excited atoms.
- Solar Cells: Devices that convert light energy into electrical energy by exciting electrons in a semiconductor material.
- Medical Imaging: Techniques like X-rays and MRI utilize the interaction of light and matter to visualize internal structures.
Conclusion: Mastering the Electron-Light Relationship
Understanding the relationship between electron energy and light is a fundamental concept in science. By mastering the principles of electron energy levels, photon energy, and the electromagnetic spectrum, and by actively engaging in learning through POGIL activities, you can develop a strong foundation in this critical area. This knowledge is essential for understanding a wide range of phenomena and technologies, from the colors of the world around us to the development of innovative energy solutions.
Frequently Asked Questions (FAQs)
1. What happens when an electron absorbs energy?
When an electron absorbs energy (e.g., from a photon or heat), it jumps to a higher energy level, moving further away from the nucleus. This is called excitation.
2. Why do different elements have different atomic spectra?
Each element has a unique atomic spectrum because the energy levels of its electrons are unique. The specific energy differences between the levels determine the wavelengths of light emitted when electrons transition between them.
3. What is the relationship between the color of light and its energy?
The color of light is directly related to its energy. Higher-energy light, like blue and violet, has shorter wavelengths and higher frequencies. Lower-energy light, like red, has longer wavelengths and lower frequencies.
4. How does a laser work?
A laser works by exciting atoms to a specific energy level. When these excited atoms release photons in a coordinated manner, they produce a highly focused and intense beam of light.
5. What is the photoelectric effect, and why is it important?
The photoelectric effect is the emission of electrons from a metal surface when light shines on it. It demonstrates the particle nature of light (photons) and is critical to understanding the interaction between light and matter, with applications in solar cells and light sensors.