Here’s an SEO-optimized article about solubility, targeting the search intent “solubility in food and drinks”:
Solubility: The Physical Property That Shapes Everything You Eat and Drink
From the satisfying crunch of a sugar crystal dissolving in your tea to the subtle release of flavors in a simmering sauce, the way substances dissolve in liquids – a property known as solubility – is a fundamental force shaping the world of food and beverages. Understanding solubility isn’t just for scientists; it’s key to appreciating the textures, flavors, and even the nutritional value of everything you consume. This article delves into the fascinating world of solubility, exploring how this physical property impacts everything you eat and drink, from the simplest cup of coffee to the most elaborate culinary creations.
What Exactly is Solubility?
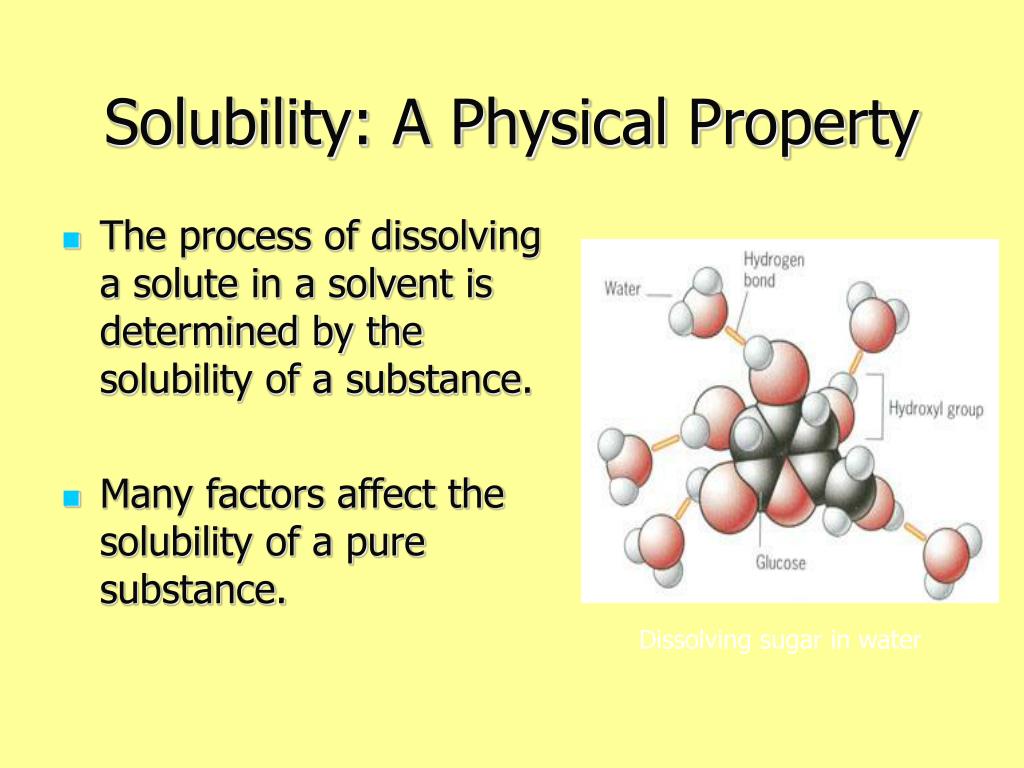
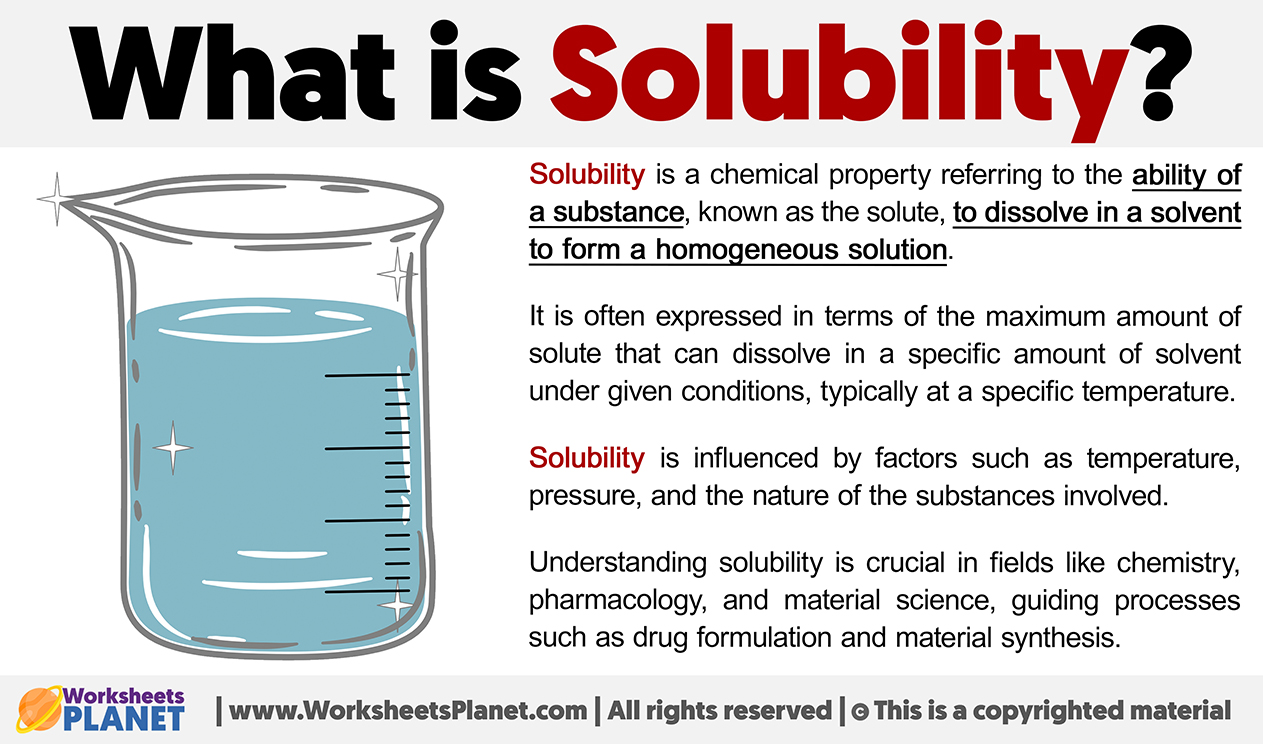
Solubility refers to the ability of a substance (the solute) to dissolve in a liquid (the solvent) to form a homogeneous mixture called a solution. Think of it like this: Imagine sugar (the solute) disappearing into water (the solvent) to create sweet tea (the solution). Not all substances dissolve equally well, and the degree to which they do is described by their solubility.
- Solute: The substance being dissolved (e.g., sugar, salt, flavor compounds).
- Solvent: The liquid doing the dissolving (e.g., water, alcohol, oil).
- Solution: The homogeneous mixture formed when the solute dissolves in the solvent.
The solubility of a substance is influenced by factors such as temperature, the nature of the solute and solvent, and pressure (especially for gases).
Solubility’s Role in Food and Beverage Production
Solubility plays a crucial role at every stage of food and beverage production, influencing everything from ingredient selection to flavor development and preservation.
- Flavor Extraction:
- Coffee and Tea: Caffeine and flavor compounds are extracted from coffee beans and tea leaves through the solubility of these compounds in hot water. The temperature of the water dramatically affects the efficiency of the extraction.
- Spices and Herbs: Cooking with spices and herbs relies on the solubility of aromatic oils and flavor molecules in fats (e.g., in curries) or water (e.g., in broths).
- Sweetening and Texturizing:
- Sugar: Sugar’s solubility in water is fundamental for creating sweetness in beverages, desserts, and sauces. Different types of sugars (e.g., sucrose, fructose, glucose) have varying solubilities, impacting the final texture and sweetness.
- Thickeners: Ingredients like starch and gelatin rely on their solubility and gelling properties in water to create the desired consistency in sauces, puddings, and other foods.
- Preservation:
- Salt Curing: Salt’s high solubility in water draws moisture out of food, inhibiting the growth of spoilage microorganisms, a key principle in meat curing and pickling.
- Sugar Preservation: High sugar concentrations, as in jams and jellies, also reduce the water activity, preventing microbial growth and extending shelf life.
- Beverage Carbonation:
- Carbonated Drinks: Carbon dioxide (a gas) is dissolved in beverages under pressure. The solubility of CO2 decreases as the temperature increases, which is why warm soda loses its fizz faster than cold soda.
- Nutrient Absorption:
- Fat-Soluble Vitamins: The body absorbs fat-soluble vitamins (A, D, E, and K) with the help of fats. Their solubility in fats is essential for their absorption in the digestive system.
How Temperature and Other Factors Influence Solubility in Food
The solubility of a substance is not a static property; it’s influenced by several factors, most notably temperature.
- Temperature: Generally, the solubility of solids in liquids increases with increasing temperature. This is why you can dissolve more sugar in hot tea than in iced tea. However, the solubility of gases in liquids decreases with increasing temperature. This is why carbonated drinks go flat faster at room temperature.
- Pressure: The pressure has a negligible effect on the solubility of solids and liquids. However, the solubility of gases in liquids increases with increasing pressure (e.g., carbonation in soda).
- Type of Solute and Solvent: The “like dissolves like” principle applies. Polar solvents (like water) dissolve polar solutes (like sugar), while nonpolar solvents (like oil) dissolve nonpolar solutes (like fats).
- pH: The acidity or alkalinity of a solution can affect the solubility of certain compounds. For example, the solubility of some minerals can be affected by the pH of the liquid.
Solubility and Food Chemistry
Understanding solubility is fundamental to food chemistry, the science that explores the chemical processes and interactions of food components. Food chemists utilize this knowledge to:
- Develop new food products: Designing recipes with the perfect balance of flavors, textures, and shelf life.
- Optimize food processing techniques: Controlling temperature, pressure, and other variables to achieve desired results.
- Ensure food safety: Understanding how solubility impacts the growth of bacteria and the stability of food components.
- Analyze food composition: Determining the amounts of different substances present in food.
Conclusion: The Ubiquitous Influence of Solubility
Solubility, the seemingly simple act of dissolving, is a powerful force that shapes the very essence of our food and beverages. From the initial extraction of flavors to the final enjoyment of a perfectly textured dish, this physical property plays a pivotal role. By understanding the principles of solubility, we gain a deeper appreciation for the complexity and wonder of the culinary world, and why the simplest cup of tea can be so satisfying.
Frequently Asked Questions (FAQs)
1. Why does sugar dissolve better in hot water than cold water?
The solubility of most solids, including sugar, increases with temperature. Higher temperatures provide the sugar molecules with more energy, allowing them to overcome the attractive forces holding them together and interact with the water molecules more effectively.
2. Why does carbonated soda lose its fizz when it warms up?
The solubility of gases, like carbon dioxide (CO2), in liquids decreases with increasing temperature. As soda warms, the CO2 becomes less soluble and escapes from the solution, causing the drink to lose its carbonation.
3. Does the type of solvent matter when dissolving something?
Yes, the type of solvent significantly impacts solubility. “Like dissolves like” is a useful principle. Polar solvents, like water, dissolve polar solutes, while nonpolar solvents, like oil, dissolve nonpolar solutes.
4. How does solubility affect the taste of food?
Solubility directly impacts taste. Flavor compounds, often present in small amounts, must dissolve in saliva (mostly water) to reach our taste buds and be perceived. The solubility of these compounds therefore influences how readily we experience the flavors of foods and beverages.