What is a Silicon Oxygen Tetrahedron? A Simple Explanation
Have you ever wondered what tiny building blocks make up the vast and varied world around us, from the sand on the beach to the gleaming glass of your smartphone screen? The answer often lies with a deceptively simple, yet incredibly versatile, molecule: the silicon oxygen tetrahedron. This fundamental structure is the backbone of a huge array of minerals and materials, playing a crucial role in everything from geology to technology. Let’s dive into what it is, how it works, and why it matters.
The Basic Building Block: What Exactly is a Silicon Oxygen Tetrahedron?
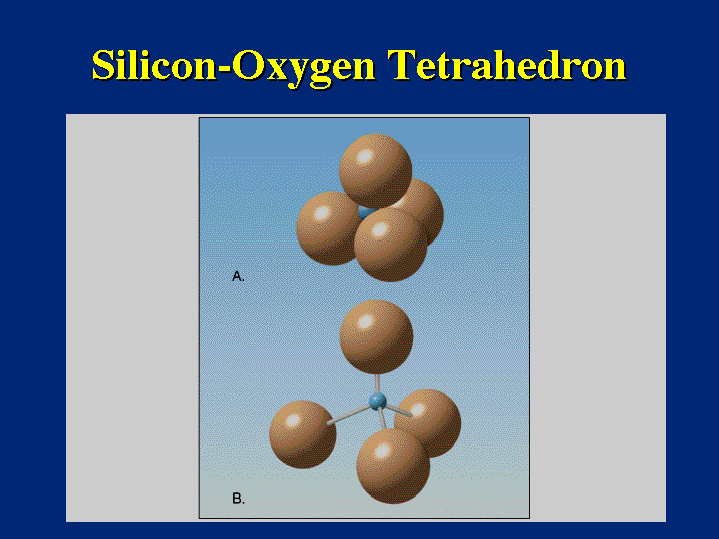
At its core, a silicon oxygen tetrahedron is a group of five atoms arranged in a specific three-dimensional shape. Picture this:
- One Silicon Atom: Located at the center. (Symbol: Si)
- Four Oxygen Atoms: Positioned around the silicon atom, forming the corners of a tetrahedron. (Symbol: O)
The silicon atom is chemically bonded to each of the four oxygen atoms. This bonding creates a strong and stable structure, much like a miniature pyramid. Think of it as a tiny, four-sided pyramid with a silicon atom at the center and an oxygen atom at each corner.
Unpacking the Structure: Key Features and Properties
Understanding the silicon oxygen tetrahedron requires a closer look at its key features and how they influence its behavior:
- Tetrahedral Shape: This specific geometry is crucial. The four oxygen atoms are arranged around the silicon atom in a way that maximizes their distance from each other, leading to inherent stability.
- Covalent Bonding: The bonds between the silicon and oxygen atoms are strong covalent bonds, meaning they share electrons. This contributes to the tetrahedron’s overall strength and resistance to breaking down.
- Negative Charge: A single silicon oxygen tetrahedron has a net negative charge (typically -4). This negative charge is due to the oxygen atoms “pulling” electrons from the silicon atom. This charge allows tetrahedra to bond with other elements and other tetrahedra, forming more complex structures.
- Versatility: The silicon oxygen tetrahedron can combine in countless ways, leading to a wide range of mineral properties.
Silicon Oxygen Tetrahedra in Action: Building Minerals and Materials
The true power of the silicon oxygen tetrahedron lies in its ability to connect with other tetrahedra and other elements. This bonding creates diverse structures that form the basis of many common minerals and materials:
- Silicates: Most common minerals are silicates, which are formed from silicon oxygen tetrahedra. Examples include:
- Quartz (SiO₂): A crystalline form of silica, with each oxygen atom shared between two tetrahedra. Found in sand, rocks, and used in glassmaking.
- Feldspar: A group of silicate minerals that are the most abundant minerals in the Earth’s crust.
- Micas: Sheet-like silicates, often used in insulation and cosmetics.
- Olivine: A mineral found in the Earth’s mantle.
- Glass: The structure of glass is essentially a non-crystalline arrangement of silicon oxygen tetrahedra, often with other elements added for specific properties.
- Cement: Cement uses silica compounds (containing silicon oxygen tetrahedra) to bind materials together.
The Importance of the Silicon Oxygen Tetrahedron
The silicon oxygen tetrahedron is vital for the following reasons:
- Earth’s Composition: It is a fundamental component of the Earth’s crust and mantle.
- Building Materials: Essential in construction, from concrete to glass.
- Technology: Used in semiconductors, electronics, and fiber optics.
- Geological Processes: Plays a critical role in weathering, erosion, and the formation of rocks.
Conclusion: The Enduring Legacy of a Tiny Structure
The silicon oxygen tetrahedron might seem like a small and simple structure, but its impact on our world is enormous. From the familiar beauty of a quartz crystal to the advanced technology in our smartphones, this tiny building block is a testament to the power of molecular architecture. Understanding its structure and properties provides a glimpse into the fascinating world of minerals, materials, and the fundamental building blocks of our planet and our technology.
Frequently Asked Questions (FAQs)
1. What is the chemical formula for a silicon oxygen tetrahedron?
The basic formula is SiO₄⁴⁻. This represents one silicon atom bonded to four oxygen atoms, with a net charge of -4.
2. How does the shape of the silicon oxygen tetrahedron contribute to its stability?
The tetrahedral shape allows the four oxygen atoms to be as far apart from each other as possible, minimizing repulsion and maximizing stability.
3. What is the difference between quartz and silica?
Quartz is a specific crystalline form of silica (SiO₂). Silica is the broader term referring to the chemical compound silicon dioxide, which can exist in both crystalline (like quartz) and amorphous (non-crystalline, like glass) forms.
4. Can the silicon oxygen tetrahedron bond with other elements?
Yes, the silicon oxygen tetrahedron can bond with a variety of other elements, especially metals like aluminum, magnesium, and iron. This bonding is what creates the diverse range of silicate minerals.
5. Why is silicon used in semiconductors?
Silicon’s ability to form strong bonds and its specific electronic properties make it an ideal material for semiconductors. It can be easily doped with other elements to control its conductivity, which is essential for creating transistors and other electronic components.